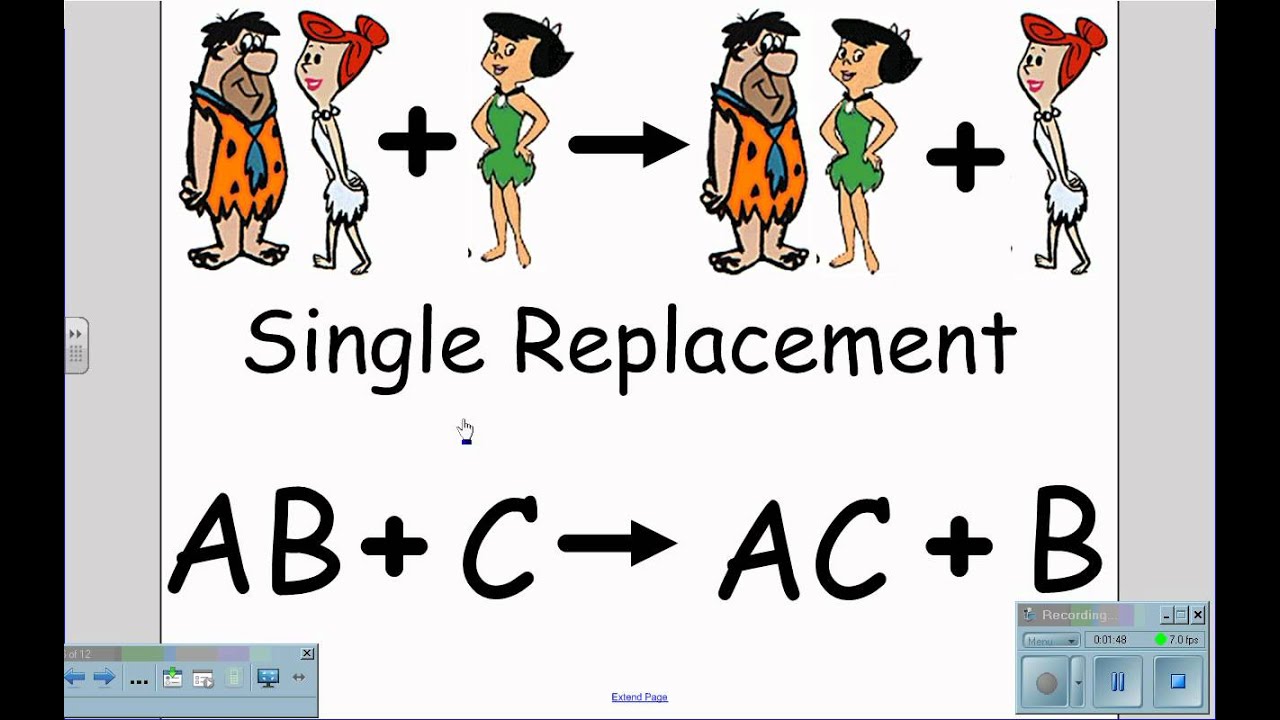
Written using generic symbols it is. 3 Single replacement reaction is a chemical change in which ONE ELEMENT replaces another element in a COMPOUND.
Classifying Chemical Reactions Flintstones Wmv Youtube
To predict whether a precipitate will form when you mix together two ionic reactants you need to know whether any of the possible products are insoluble.
. A double-replacement reaction sometimes referred to as a double-displacement reaction occurs when parts of two ionic compounds are exchanged making two new compounds. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. Neutralization precipitation and gas formation are types of double.
The displacement reaction is a type of REDOX reacn. Here is another way to look at the above generic example. A precipitate is an insoluble solid compound formed during a chemical reaction in solution.
AB becomes AD by giving up B to take up D from CD. The double replacement reaction occurs when ionic compounds react in aqueous solutions resulting in the formation of other ionic compounds. DOUBLE REPLACEMENT PRACTICE REACTIONS For each reaction predict the products and balance the equation.
When you are finished draw your own comic strips to illustrate the following reaction types. 5 Combustion reaction is a chemical change in which an element or a compound reacts with oxygen often producing energy in the form of heat and light. Cartoon Chemistry and Reaction Types Directions.
Usually the solvent is water. Single replacement reactions can be recognized because both the reactant and the product have an element and a compound 4 Double-replacement reaction is a chemical change involving an exchange of positive ions between two compounds. A double replacement reaction is a type of chemical reaction.
One of the factors driving a double replacement reaction is the formation of a solid precipitate. A double displacement reaction is also called a double replacement. In a double replacement reaction the reaction proceeds according to this general form.
State the reaction in chemical formulas and in symbols. The reaction takes place between two ionic. AB CD AD CB.
CARTOON CHEMISTRY Types of Chemical Reactions Name. Double-replacement reaction is a chemical change involving an exchange of positive ions between two compounds. When a decomposition reaction occurs when one reactant breaks down into several products.
Imagine a hypothetical situation where a kid who loves ice cream is going out with his two friends. Create an illustrationcartoon example for each of the 4 main types of reactions. During double replacement the cations and anions of two different compounds switch places.
It must be noted that the results are not 100. The chemical bonds between the reactants may be either covalent or ionic. This is simply based on the solubility chart of inorganic compounds.
For example when silver nitrate combines with sodium chloride two new compounds--silver chloride and sodium nitrate are formed because the sodium and silver switched places. In double replacement reactions the swapping of elements takes place. Select two compounds above and this calculator will predict whether or not the reaction will occur in water.
1st a product is slight soluble and precipitates 2nd one of the product is a gas and 3rd one product is water. A double replacement reaction will occur if a formation of a precipitate gas or water takes place. For the following reactions use the Activity Series of Metals to determine if a reaction will occur and if so what the products will be.
AB XY --- AY XB. _____ Describe the chemical reaction illustrated below each diagram. No DR Reaction A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
Double replacement reactions may involve reactants that contain either ionic or covalent bonds but the type of reaction is more common with ionic compounds. For example the formula HCl can be used for hydrogen chloride gas HClg so to indicate hydrochloric acid one must specify HClaq. Double displacement reactions take the form.
Acids and bases may participate in double replacement reactions. This edutainment video represents the single replacement reaction in a very attractive way. CD then accepts B from AB to become CB.
Double replacement reaction chemistry worksheet answer 50 examples of balanced chemical equations. The name describes the process very well. The overall pattern of a double replacement reaction looks like this.
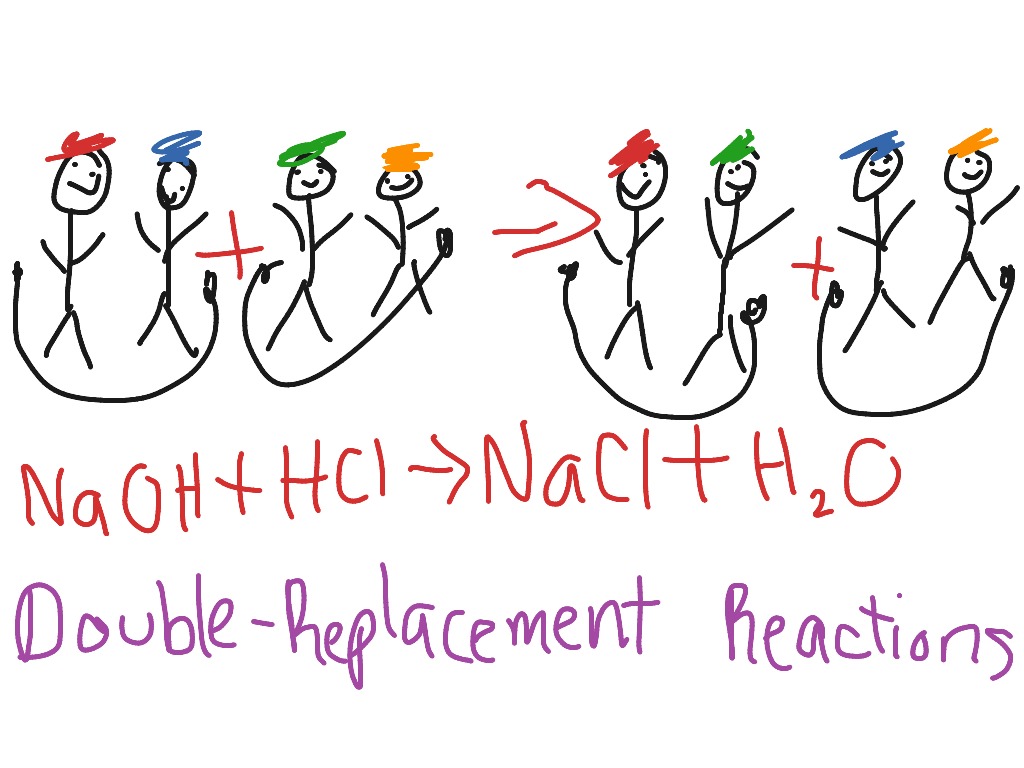
AB CD AD CB. The displacement reaction is a type of REDOX reacn. AgNO 3 NaCl --- Silver nitrate sodium chloride silver chloride sodium nitrate AgNO 3 NaCl --- AgCl NaNO 3 1 CaOH 2 H 3 PO 4--- 2 K 2 CO 3 BaCl 2--- 3.
In this reaction ions show replacement. Double Replacement Reactions Fall 2017 page 4 of 9 Note that the physical state aqueousaq must be included to distinguish the acid from other forms of a substance. This is a reaction between two ionic compounds in which the two swap partners in this way.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products. This Edutainment video represents the Single Replacement Reaction in a very attractive way.
Na 1 aq Br 1- aq H 1 aq Cl 1- aq. Initially he is holding hands with Friend A but Friend B soon joins. The reactants AB and CD each give up their partners to the other.
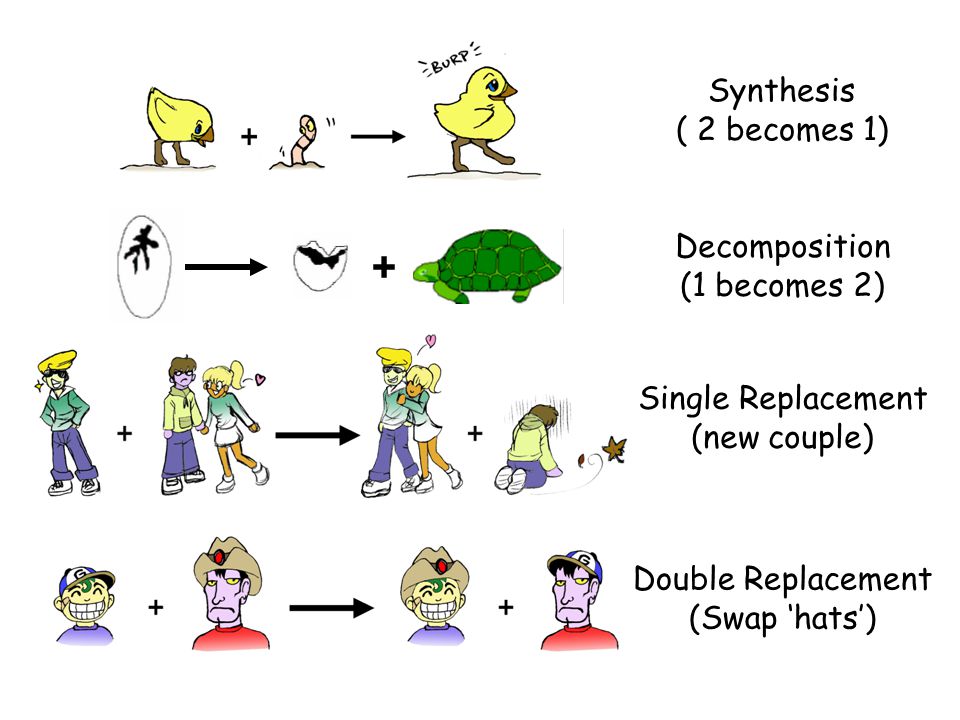
Synthesis decomposition single replacement double replacement. By tracking the positions of cations and anions in reactants and products one can find whether the double displacement reaction occurs or not. Two replacements take place in the reaction.
Reactant reactant ----- product product To visualize a double replacement reaction look at the following cartoon. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. Use uppercase for the first character in the element and lowercase for the second character.
Double Replacement Reaction Model - Http Www Loreescience Ca Uploads 2 4 1 7 24170983 Cartoon Chemistry And Reaction Types Assignment Nov 2017 Pdf - The reaction in which two compounds react together to form two other compounds by mutual exchange of their ions is called double displacement reaction. For each of the 5 types of reactions on the back of this paper. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
Agno 3 nacl agcl nano 3 4 double displacement 1 double replacement 2 single replacement 3 decomposition 4 synthesis 2. Double replacement reaction cartoon. A and X are the cations postively-charged ions in this example with B and Y being the anions negatively-charged ions.
Usually a double displacement reaction results in precipitate formation. The chemical equation for this double replacement reaction looks like. For a double-replacement reaction to occur one of the following must occur.
Fe au co br c o n f.

Cartoon Chemistry And Reaction Types

Dublin Schools Lesson Types Of Chemical Reactions

Double Replacement Reaction Ppt Download

5 2 Reaction Types B Double Replacement Combustion Youtube

0 comments
Post a Comment